QMS Document change control is resource intensive, time consuming, prone to audit findings, here are our top 5 findings.


1. For Document Change Control, approval requirements for different document types are different.
In the QMS, you will have many document types including SOP(standard operating procedures), forms, work instructions, specifications. As per the regulatory requirements, the documents are to be reviewed and approved by originating functions, or the approval should be done by equivalent technical expertise and authority (21CFR820.4 (b) Document changes. Changes to documents shall be reviewed and approved by an individual(s) in the same function or organization that performed the original review and approval, unless specifically designated otherwise. ) Documents (SOPs, Specs, Instructions) for individual functions may need specific group of approvers, e.g.
• QA Documents may require approval from, QA, OPS, and R&D
• R&D Documents may require approval from R&D and QA
How do you ensure the document types aligns with approval requirements?
• Quick Solutions: We always look for quick solutions to fix the issues, which creates unintended issues further down the line. For example, to manage the approval requirements, we create checklist for approval requirements, ensure the checklist is followed when making changes. (we all know how good are manual processes, often time someone forgets, some misses and so on..)
• What happens when someone forgot to follow the checklist or missed it? There can be some audit finding. Then more training and monitoring of the people and process.
Better Solutions:
Use of Approval Matrix For Document Change Control: The approval matrix option in Qualcy eQMS helps enforce review of the DocFolders through change requests (CR) by the selected functional roles in the company. The approval matrix can be created by users with Admin role. It is a prerequisite to create the approval matrix, prior to create any DocFolder in the DOC MGMT module. Once the approval matrix is created, this would be available for future use.
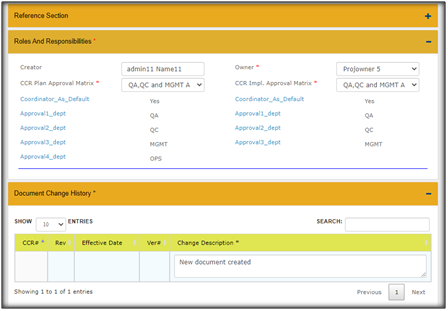

Here are the simple steps to create an Approval Matrix in Qualcy eQMS:
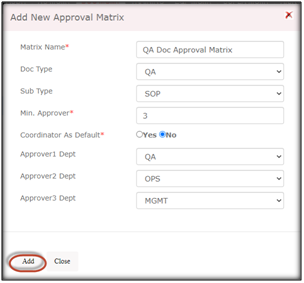

Enter the name for Approval Matrix, Document Type and Subtype, Minimum number of approvers, select the Approver department names, and click Add, this will make the approval matrix active and be visible in the list. You can create multiple Approval Matrix for different Document types and Subtypes.


2. Tracking the changes and redlines are difficult.
The document change control requires teamwork and collaborations. Here are some of the issues that are challenging.
• How do you prevent others making the changes at the sametime
• How do you ensure changes, redlines copies are available and traceable to person making the change?
• When changes are being made, who is doing the impact assessment? How are the related documents, specs being handled?
• How do you store and retain the different versions of records and documents?
Quick Solution:
Use manual process and resources (do not forget, that person has to be trained and monitored). In a typical manual process, when you want to revise a document, you will request a copy from the document controller. You will make the edits, you will print out the copies of the redlined documents. You will attach the redlined documents to the change request form. You will forward the package to the next collaborator. This is repeated by other collaborators who are working on the same documents. Once the changes are completed, you will send the final package for review by the approvers. If there are 3 or 4 people are involved, this could easily take more than a month. Because people have other priorities on the desk. (The other reason, it takes longer for the manual document change control, it is labor intensive and boring. So, people tend to procrastinate, which makes it more challenging)
Better Solutions:
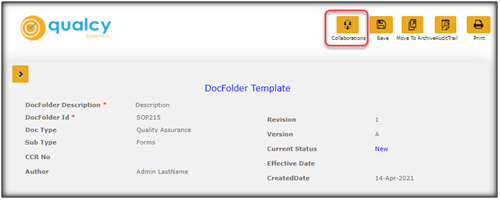
Use Automated Collaboration Option for Document Change Control. The DocFolders in Qualcy eQMS provide option for Collaboration with other users. Under Collaborations, the user can Share as Link, Check Out, Check In, Undo Check Out, Enable Collaboration, Disable Collaboration, Send for Approval, Review, Send For Training, and Make Status Change.
Here are some simple steps to create the collaboration option in the Qualcy eQMS.
From the menu options, click on the Collaborations button then select Enable Collaboration. The window shall refresh and Check Out, Disable Collaboration, Share as Link, and Send for Approval shall be enabled as selection options under Collaborations.



3. Approval cycle times are long.
Probably you have heard it, it takes forever to get something changed and released. Here are the issues we have seen.
· Use of office mails and envelops to move the documents from one person to another (yes, we are in 21st century and age of internet, unfortunately, it is a common practice in lot of places)
· Find out who has it, send them weekly emails, and call them if you would need something urgently.
Quick Solution:
You can have someone track the check outs and send emails and do the follow ups. Though this is not the best solution, most companies rely on human interface for the tracking and reminders.Sometimes it gets complicated when the emails are escalated to the next level managers. Then it impacts team moral.
Better Solution:
Use electronics Change Request Templates for Document Change Control Content Change Request (CCR): In the Qualcy eQMS the CCR is the vehicle to control (or communicate) the change requests in a streamlined process. CCR takes the request for change of DocFolder(s) from the Author/Owner to the Approvers. CCR provides options to Project Owners to expedite the tasks done by the Task Owners. CCR allows Approvers to approve or reject the change requests. CCR allows movement of DocFolders to respective cabinets depending upon workflow. The Released DocFolders move to Released Cabinet, or the Retired DocFolders move to Archived Cabinet, when the change requests are approved. The details of the Document change history is established when CCR is used.
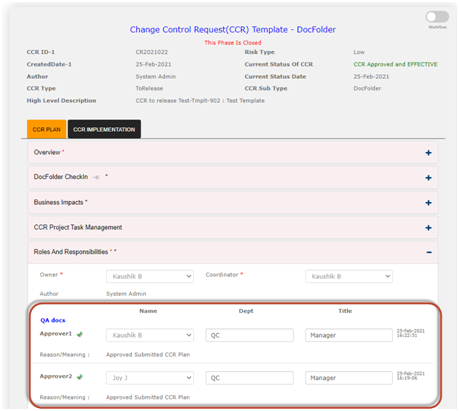

· The CCR workflow for Document change control is completed in two phases. The first phase is review and approval of the “CCR plan” and second phase is the implementation of the approved CCR plan which is referred as “CCR Implementation” in the Qualcy EQMS. The workflow for both the phases are reviewed and approved for successful completion of the phases. The current status provides information regarding the status of the CCR during the workflow. The current status of CCR can only be changed by the System workflow.
· In the automated system, then there is no problem with sending reminders. As per your set up, the automated system sends out 3 reminders to the user and then 4th one goes to next level manager. People know this in advance, so they take proactive actions.


4. Implementation of the approved changes are challenging.
Yes, the change request got approved, now what? Implement the changes. Here are the common issues.
(21 CFR820.4 (b) Approved changes shall be communicated to the appropriate personnel in a timely manner. Each manufacturer shall maintain records of changes to documents. Change records shall include a description of the change, identification of the affected documents, the signature of the approving individual(s), the approval date, and when the change becomes effective.)
· Send for review and get the training records signed offs. How?
· Use office mails and envelops (yes, we use pen and paper, very common in many places)
· Send email reminders and follow ups, when it gets delayed.
· Verification and Validation of the changes
· Verification and validation of changes are required prior to implementation of the changes to minimize the adverse impacts.
· Notification of changes
· Send notifications to suppliers, customers and other parties.
· Submit regulatory filings
Quick Solutions:
You can use manual process to keep records for the above notifications and implementations. In a typical manual process, you will need a designated person to coordinate the implementation of the changes. Make sure the notifications have been properly done. Then collect the records and file it with the change request file.
Better Solutions:
CCR Implementation Task Management: In Qualcy eQMS there is option for managing tasks that are required for implementation of Document change control. Under this section, new tasks can be created and released to the task owners. All tasks created under this section are saved here. There are links available here to open the previously created tasks.
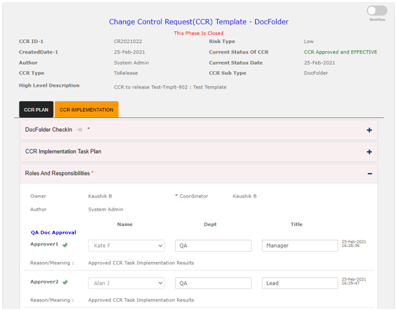

If there is any need to notify the customers or the suppliers, you will complete those tasks in this phase. Also you can created new Training assignments. In this case the new Training assignment ID will be added under the CCR implementation task table. The CCR number will be linked to the Group Training Assignment created inside the Training Module.


5. Doing the periodic reviews is error prone.
For QMS Document Change control:
You must review the released and effective documents in regular intervals to ensure, the processes are still valid. Here are some common issues.
· Create a list of documents and assign owners
· Send emails and follow ups to get the reviews done by the due date
· Monitor the metrics using Excel
Quick Solutions:
In a typical manual process, you will need a designated person to coordinate the periodic review of the documents. If something gets missed, it is usually blamed as human error. If there is audit finding, then this is also classified as human error. More training and monitoring are used as solutions.
Better Solutions:
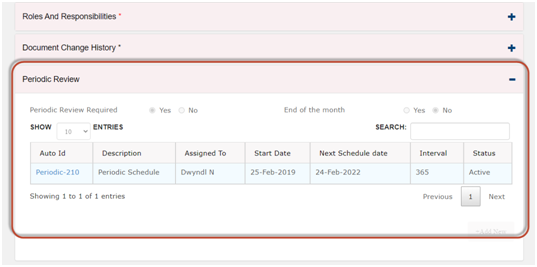
Use automated review schedule to manage the requirements for Periodic Review.
· In Qualcy eQMS, there is option for setting up auto schedule to manage the requirements for the periodic review. The assigned to users can be notified 30, 60 or 90 days in advance as per the reminder set up. The users can document the completion of the reviews with eSign and comments.
· The metrics for the coming due reports are auto generated by the system.
We have posted another blog post to help you create a detail procedure to manage the QMS Document Change Control.