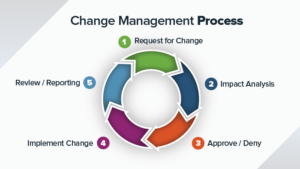
NC Management System
We provide complete solution for handling of process and product non conformances. Following are some of the key features of the NC Management solution.
- Easily create NC records, communicate with NC owners and Approvers
- System provides reminders for Reviewers and Task Owners.
- The system has role based identification and log in and access control, which is very secured and convenient for use. Tasks or project will not wait for someone on a vacation…
- Workflow inbox capabilities provide easy visibility of notifications, tasks, and projects at a single place. You only see your tasks and notifications.
- The number of approvers can be different for each project, or you can assign minimum number of approvers and departments the approvers should be from.
More about our solution for NC Management System
We do not live in perfect world. This does not mean we cannot aspire to achieve near perfection. So we need to properly handle and document the NC scenarios whether it is a process nonconformance or a product nonconformance. We need to monitor and analyze the underlying root causes. We need to assign and implement appropriate corrective actions so that these NCs never reoccur. That is when we would approach near perfection. The customers would not only be satisfied but delighted to recommend our products and services to others.
So we have created a system that will be easy to implement and easy to use. We want to change the way the Quality systems are implemented and used. It does not have to be – for compliance only purpose, it does not have to be- this is how the system works. We have created solutions that is efficient and effective. This can be customized as per your process requirements. The system is flexible enough to include all your requirements and still be user friendly and intuitive. Above all, your processes will comply with the ISO standard, 21CFR part 820 for medical devices, AS standards or other industry standards or regulations.
Highlights of the Features and Capabilities
- The system has role based identification and log in and access control, which is very secured and convenient for use. Tasks or project will not wait for someone on a vacation…
- Workflow inbox capabilities provide easy visibility of notifications, tasks, and projects at a single place. You only see your tasks and notifications.
- The number of approvers can be different for each project, or you can assign minimum number of approvers and departments the approvers should be from.
- The System has E-signature capability that is auditable and traceable
- Pdf printing capability with time stamps, and individual traceability
- Can attach as many files and any type of files. The records can be created using any type of files including audio and video files.
- No more painful scrolling or small text boxes…The users enjoy the benefits of drag and read text boxes. You can drag the boxes to read as much content you need.
- The project owners can assign tasks; follow up updates until the tasks are completed.
- The system provides automated reminders for the task owner, project owners , approvers as per custom configurations
- The system provides options for creating metrics and reports. Export the data to Excel and create customized reports as needed, for management review, monthly, quarterly, or annual performance metrics.
- Option for configurable labels for the text boxes. You do not have to accept- this is how the system works.