QA/RC Consulting
We provide complete solution and service for managing the Quality Management and Regulatory Compliance for Medical Device, Biotech and Pharma Companies.
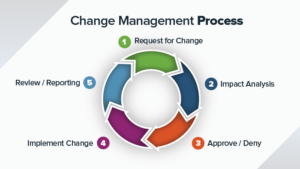
This includes QMS set up, maintainance and improvement. We also provide Regulatory compliance management services including 510K filing, IND, ANDA, DMF filing and others.
Followings are key features and capabilities of our QA/RC consulting service options.

- Creating Documents and Records
- Providing QMS support, employee trainings
- Managing audits(Internal, Supplier, Regulatory)
- Supplier Qualification and Approval
- Design history files and risk managements
- Regulatory filings
More about our QA/RC Consulting Solutions
We have team of experts who are available for helping our customers. Our services combined with software platform, enables our customers scale up opearations and launch products faster to market. We support our customers end-to-end so that they can go to market faster with a small team. We help reduce the risk involved in the new product launches.


Highlights of the Features and Options
Our consultants help our customers estblish QMS systems from scatch. We provide training services to enable our customerunderstand the functionalities of the QMS and be capable of running the QA/RC function independently.
The followings are highlights of our eQMS platform:
There are other systems available in the market which are not flexible or user friendly. We have developed a system that is easy to implement and easy to use. The system has built in features that enable compliance with regulatory requirements such as ISO 9000, 21CFR Part820, 21CFR part 211.
- The number of approvers can be different for each project, or you can assign minimum number of approvers and departments the approvers should be from.
- The System has E-signature capability that is audit-able and traceable
- Pdf printing capability with time stamps, and individual traceability
- Can attach as many files and any type of files. The records can be created using any type of files including audio and video files.
- No more painful scrolling or small text boxes…The users enjoy the benefits of drag and read text boxes. You can drag the boxes to read as much content you need.
- The system provides automated reminders for the task owner, project owners , approvers as per custom configurations
- The system provides options for creating metrics and reports. Export the data to Excel and create customized reports as needed, for management review, monthly, quarterly, or annual performance metrics.
- Option for configurable labels for the text boxes. You do not have to accept- this is how the system works.