Blogs
Leverage Power of Information and Exceed Customer Expectation
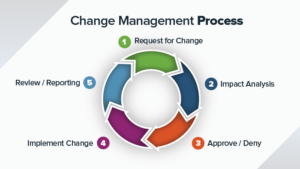
Corrective and Preventative Action (CAPA)...
It is challenging to manually manage Corrective and Preventative Action (CAPA) process with paper based or Excel file system. Here…
What is Design Control of...
The Design Control of Medical Devices includes but not limited to creation, change and reviews of design data, including but…
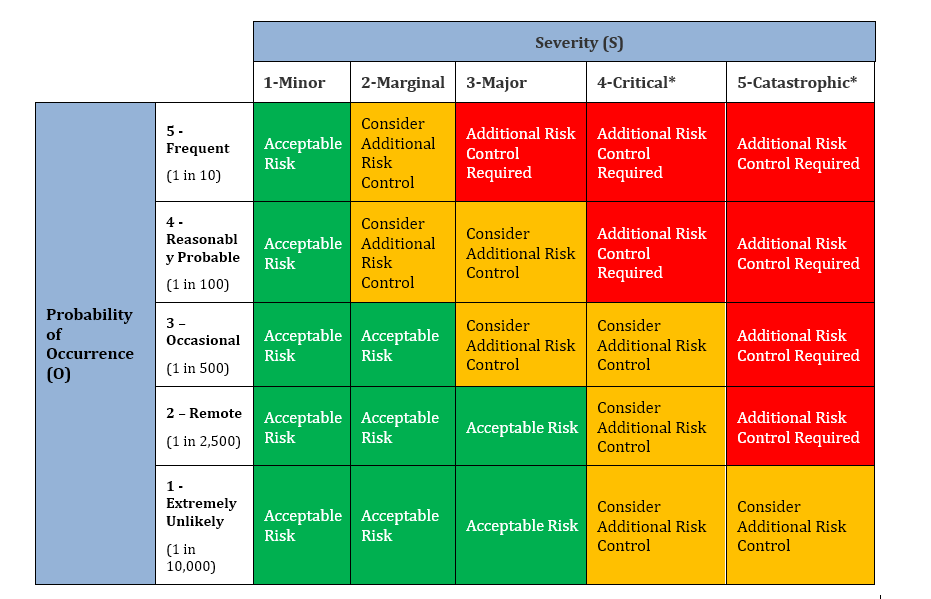
Medical Device Quality Risk Management
Risk Management in QMS and New Products Design (NPD) from Sanjay Dhal , MS, MBA By adding quality risk…
QMS Software for ISO13485
There are many challenges involved when implementing a QMS system that will comply with ISO13485:2016. Here are top 10 requirements…
Top 10 Factors for Selecting...
TOP 10 FACTORS TO CONSIDER FOR SELECTING QMS Software For Pharma and Life Science Companies There are many challenges involved…
Top 10 Factors for Selecting...
TOP 10 FACTORS TO CONSIDER FOR SELECTING QMS Software For Medical Device and Pharma Companies. There are many challenges involved…
Top 5 issues with Document...
QMS Document change control is resource intensive, time consuming, prone to audit findings, here are our top 5 findings. 1.…
Supplier Quality Management - How...
Effective Supplier Quality Management – How to do this? How to establish an effective supplier quality management system? Why is…
Internal Audit Management, Policy and...
Internal Audit Management- How to do this? The Internal Audit Policy and Procedure describes the internal auditing process to evaluate…
Calibration Management
If you are running a calibration management operation with paper based or Excel spreadsheets, it may be taking lot of…
Document Change Control for QMS
The Document Change Control is one of the key requirements for ISO9001, ISO13485 and other ISO standards. Effective Document Change…
Employee Training Program
Why do we need to train employees? Why do we need to keep training records for employee training program? Managing…
Why do we need Quality...
Risk Management in QMS and New Products Design (NPD) from Sanjay Dhal , MS, MBA By adding quality risk…
Calibration and Asset Management in...
The Cal.ibration and Asset Management is one of the key requirements for ISO9001, ISO13485 and other ISO standards. Effective Calibration…
How To Select a Better...
If you are running a Calibration, Preventative Maintenance Management, Asset Management operation with paper based or Excel spreadsheets, it may…
Simple Guide for Corrective and...
The following section describes a sample SOP (standard operating procedure) for corrective and preventive actions (CAPA) in your Quality Management…
Simple Guide for ISO13485 Auditing
This document provides simple guidance and details you would need to set up your internal auditing process to comply with…
Information Center